In the News... FDA approves Tandem Mobile Bolus, Eversense 180 Day Sensor, and more! - Diabetes Connections

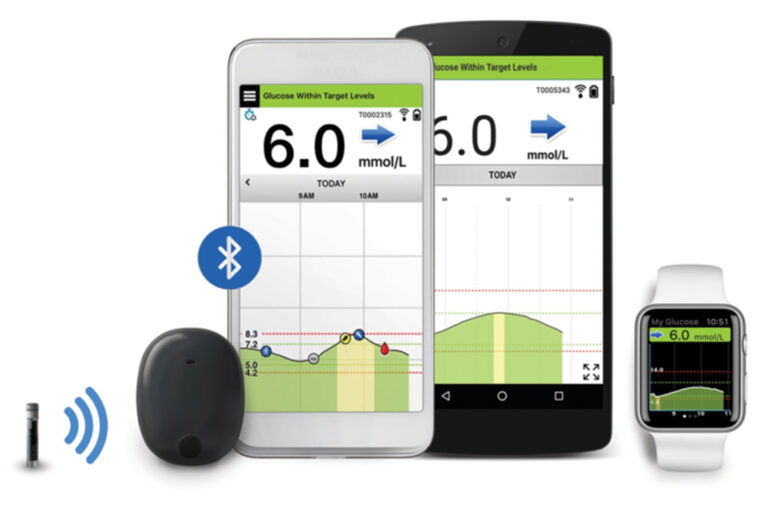
ASCENSIA DIABETES CARE ANNOUNCES FDA APPROVAL OF THE EVERSENSE E3 CONTINUOUS GLUCOSE MONITORING SYSTEM FOR USE FOR UP TO 6 MONTHS

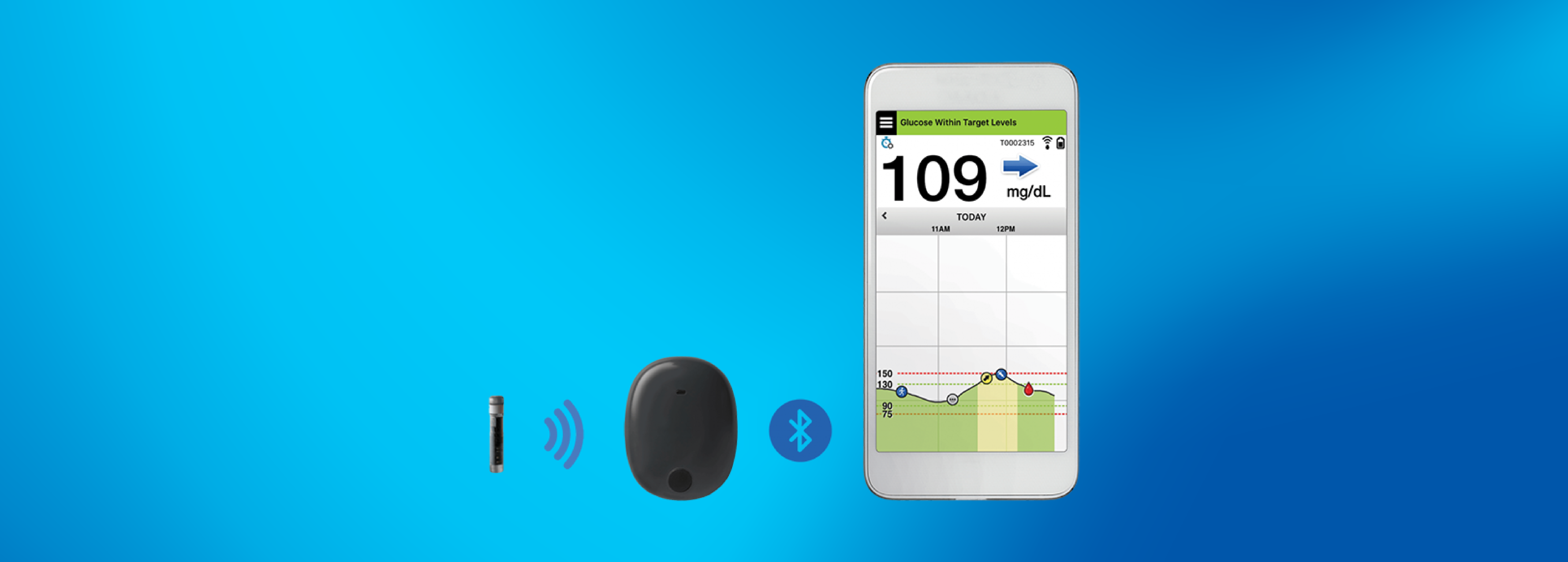
Beyond Type 1 - NOW APPROVED BY THE FDA - a new CGM is on the scene! The Eversense from Senseonics is the first implantable continuous glucose monitor. It lasts 90 days

Eversense Implantable Glucometer Gets FDA Approval as Alternative to Finger Tests - 20451 Seneca Meadows Pkwy, Germantown, MD 20876, USA

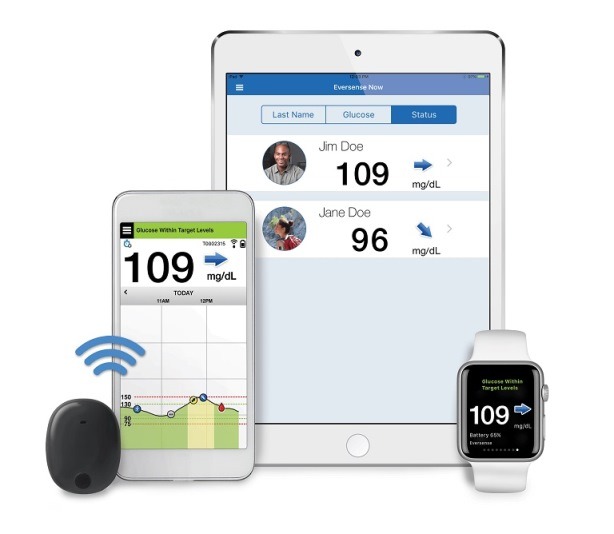
Senseonics Receives FDA Approval To Expand Eversense CGM Certification To Nurse Practitioners, PAs | Medical Product Outsourcing


![6-Month Glucose Implant Sensor Receives CE Mark Approval [video]|Health Tech Insider 6-Month Glucose Implant Sensor Receives CE Mark Approval [video]|Health Tech Insider](https://i0.wp.com/healthtechinsider.com/wp-content/uploads/Eversense_CGM_with_video_600x275.jpg?fit=600%2C276&ssl=1)